Myeloproliferative Neoplasm (MPN) is a group of chronic blood cancers characterized by the somatic acquisition of mutations, in particular Janus Activated Kinase 2 (JAK2V617F), which lead to constitutive activation of myeloid cytokine receptors. Interferon alfa (IFNα) is the only known therapy that induces a molecular response by decreasing the Variant Allele Frequency (VAF) of JAK2V617F (Mullally et al, Blood 2013; Mosca et al, Blood, 2021). However, not all MPN patients on IFNα enjoy a molecular response, and why some patients respond and others do not remains unclear. Our long-term goal is to identify early biomarkers that predict for a molecular response to IFNα by following in depth MPN patients who are beginning IFNα dosing for one year with daily sampling of leukocyte DNA to monitor day-to-day changes in the driver mutation VAF using digital PCR (dPCR) (Cordua et al, Blood, 2019).
Biosample collection and processing: peripheral blood was drawn into an EDTA tube and aliquoted into 500 µL stocks. Saliva was collected via passive drool and Zymo DNA/RNA Shield was added in a 1:1 dilution.
DNA purification: DNA was purified using the Zymo Research Quick-DNA Miniprep Plus Kit and DNA yields (ng/µL) were quantified using the Qubit Flurometer.
dPCR: DNA was added to master mix, JAK2WT and JAK2V617F specific primers and fluorescent probes, and sterile water. dPCR protocol: 10-minute pre-heat at 96°C, followed by 45 cycles of denature at 96°C for 30 seconds/anneal-extend at 61°C for 60 seconds, analyzing for JAK2WT and JAK2V617F fluorescent signals to quantify the amount of DNA (cp/µL) per allele. VAF was calculated using the amounts of DNA (JAK2V617F)/(JAK2WT+JAK2V617F).
First, using stored samples from patients received before and during IFNα treatment, we identified variable responses of the JAK2V617F VAF to IFNα. We observed that patients with a positive clinical response to IFNα had an increase in JAK2V617F VAF immediately after initiating this therapy, followed by an eventual decrease.
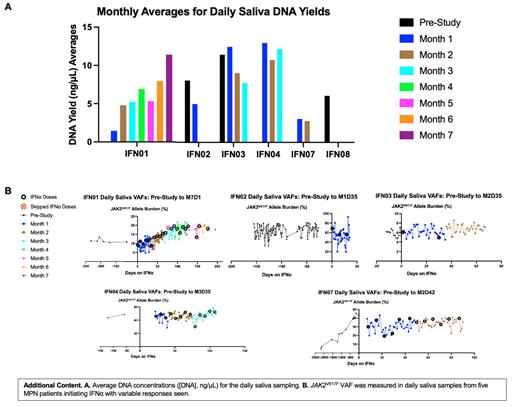
Then, we showed the feasibility for a study using daily saliva sampling in an IRB-approved observational trial to monitor daily VAF trends via saliva sampling for one year. Participants provided daily sampling starting from ~1 month before initiating IFNα therapy. So far, we have recruited eight patients. For most patients, high DNA yields (> 5 ng/µL) can be consistently obtained (Additional Content A). For one patient (IFN01) with initial low yields, we were able to improve those yields with positive reinforcement (e.g., smelling lemon-scented stickers). Next, we view that most patients can maintain consistent daily sampling times and food/drink conditions.
Using the daily sampling, we have developed JAK2V617F change curves in response to IFNα (Additional Content B). There is daily variation in VAFs (hypothesized to be from variable buccal epithelial contamination), however clear trends can be seen. One patient (IFN01) displays a steep increase in JAK2V617F VAF, another (IFN07) displays a more gradual increase, and two patients (IFN03, IFN04) display no noticeable change. These trends will be interesting for continued observation to determine if they will correlate with long-term outcome IFNα.
From the above graphs, after viewing that weekly sampling (which for most patients are symbolized by the black circles for weekly IFNα injections) preserves the same VAF trends as daily sampling, we simulated 1000 weekly, bi-weekly and monthly data distributions by randomly selecting a data point from each consecutive time frame over the first 150-day period, respectively. We will then conduct a generalized linear model to construct a plot of predicted mean values across time and its 95% confidence intervals (CIs). We determined that moving forward, we can decrease the sampling frequency to weekly samples, since this will preserve fine detailed trends, as well as have the combination of ease for patient and frequent enough for patients to not forget sampling habits.
Here, we explore the novel use of daily saliva for comprehensive and high resolution VAF tracking of MPN patients in a remote setting. We will continue to follow the day-to-day variation of VAF in response to IFNα and plan to expand the use of saliva to gene expression profiling. Overall, the results of this trial will be used to generate a preliminary model of IFNα response, which can be used to predict how likely a MPN patient will benefit from IFNα therapy.
Disclosures
Fleischman:Pharmaessentia, CTI: Speakers Bureau; GSK, Incyte, CTI: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal